Ruthenium, osmium and rhodium complexes of 1,4-diaryl 1,4-diazabutadiene: radical versus non-radical states†
Abstract
Ruthenium, osmium and rhodium complexes of 1,4-di(3-nitrophenyl)-1,4-diazabutadiene (LDAB) of types trans-[RuII(LDAB)(PPh3)2Cl2] (1), trans-[OsII(LDAB)(PPh3)2Br2] (2) and trans-[Rh(LDAB)(PPh3)2Cl2] (3) are isolated and characterized by elemental analyses, IR, mass and 1H NMR spectra including the single crystal X-ray structure determination of 1·2toluene. The α-diimine fragment of the LDAB ligand in 1·2toluene is deformed, showing a relatively longer –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bond, 1.320 Å, and a shorter
N– bond, 1.320 Å, and a shorter ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH
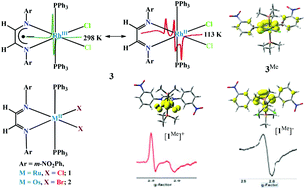
CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) bond, 1.395 Å. Density functional theory (DFT) calculations on trans-[Ru(LDAB)(PMe3)2Cl2] (1Me) and trans-[Os(LDAB)(PMe3)2Br2] (2Me) with singlet spin states authenticated that the closed shell singlet state (CSS) solutions of 1 and 2 are stable and no perturbation occurs because of the diradical open shell singlet (OSS) state. The EPR spectra of 3 and the Mulliken spin density distribution obtained from the DFT calculation on trans-[Rh(LDAB)(PMe3)2Cl2] (3Me) imply that the ground electronic state of 3 can be defined by the [RhIII(LDAB˙−)(PPh3)2Cl2] (3RhL˙) ↔ [RhII(LDAB)(PPh3)2Cl2] (3Rh˙L) resonating states. In solid, the contribution of 3RhL˙ is higher and the gav is 2.018 with Δg = 0.10, whereas in frozen glasses the contribution of 3Rh˙L is higher and the gav is 2.026 with Δg (frozen glasses) = 0.13. The g parameters of the electrogenerated [1]+ (g1 = 2.456, g2 = 2.128 and g3 = 1.624, Δg = 0.824), [2]+ (g1 = 2.599, g2 = 2.041 and g3 = 1.965, Δg = 0.634), [1]− (g1 = 2.138, g2 = 2.109, g3 = 1.978 and Δg = 0.160) and [2]− (g1 = 2.168, g2 = 2.097, g3 = 1.987 and Δg = 0.181) ions and the spin density distributions obtained from the DFT calculations on [1Me]+, [2Me]+, [1Me]− and [2Me]− reveal that the reversible anodic peaks of 1 and 2 at 0.11 and 0.34 V, referenced versus Fc+/Fc couple, are due to the M(III)/M(II) redox couple, while the reversible cathodic waves at −1.27 V and −0.82 V of 1 and 2 are caused by the LDAB/LDAB˙− redox couple. Both [MII(LDAB˙−)(PPh3)2Br2]− and [MI(LDAB)(PPh3)2Br2]− tautomers contribute to the ground electronic states of [1]− (g = 2.075) and [2]− (g = 2.084) ions, which are isoelectronic to 3. Time dependent (TD) DFT calculations and spectroelectrochemical measurements elucidated that lower energy absorption bands of 1 and 2 are caused by the metal to ligand charge transfer (MLCT) that disappears upon oxidation or reduction.
bond, 1.395 Å. Density functional theory (DFT) calculations on trans-[Ru(LDAB)(PMe3)2Cl2] (1Me) and trans-[Os(LDAB)(PMe3)2Br2] (2Me) with singlet spin states authenticated that the closed shell singlet state (CSS) solutions of 1 and 2 are stable and no perturbation occurs because of the diradical open shell singlet (OSS) state. The EPR spectra of 3 and the Mulliken spin density distribution obtained from the DFT calculation on trans-[Rh(LDAB)(PMe3)2Cl2] (3Me) imply that the ground electronic state of 3 can be defined by the [RhIII(LDAB˙−)(PPh3)2Cl2] (3RhL˙) ↔ [RhII(LDAB)(PPh3)2Cl2] (3Rh˙L) resonating states. In solid, the contribution of 3RhL˙ is higher and the gav is 2.018 with Δg = 0.10, whereas in frozen glasses the contribution of 3Rh˙L is higher and the gav is 2.026 with Δg (frozen glasses) = 0.13. The g parameters of the electrogenerated [1]+ (g1 = 2.456, g2 = 2.128 and g3 = 1.624, Δg = 0.824), [2]+ (g1 = 2.599, g2 = 2.041 and g3 = 1.965, Δg = 0.634), [1]− (g1 = 2.138, g2 = 2.109, g3 = 1.978 and Δg = 0.160) and [2]− (g1 = 2.168, g2 = 2.097, g3 = 1.987 and Δg = 0.181) ions and the spin density distributions obtained from the DFT calculations on [1Me]+, [2Me]+, [1Me]− and [2Me]− reveal that the reversible anodic peaks of 1 and 2 at 0.11 and 0.34 V, referenced versus Fc+/Fc couple, are due to the M(III)/M(II) redox couple, while the reversible cathodic waves at −1.27 V and −0.82 V of 1 and 2 are caused by the LDAB/LDAB˙− redox couple. Both [MII(LDAB˙−)(PPh3)2Br2]− and [MI(LDAB)(PPh3)2Br2]− tautomers contribute to the ground electronic states of [1]− (g = 2.075) and [2]− (g = 2.084) ions, which are isoelectronic to 3. Time dependent (TD) DFT calculations and spectroelectrochemical measurements elucidated that lower energy absorption bands of 1 and 2 are caused by the metal to ligand charge transfer (MLCT) that disappears upon oxidation or reduction.

 Please wait while we load your content...
Please wait while we load your content...