Carbazole substituted boron dipyrromethenes†
Abstract
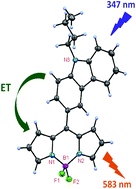
meso-Substituted BODIPY with N-butylcarbazole (1) was prepared and derivatized. Dibromo BODIPY 2, α-formyl BODIPY 3 and β-formyl BODIPY 4 were synthesized. All compounds were characterized by HRMS, NMR, UV-vis absorption, electrochemical and fluorescence techniques. The crystal structures of BODIPY 1 and its dibromo derivative 2 were also solved. In both the X-ray structures, the dihedral angle between the meso-carbazole group and the dipyrrin plane was decreased, suggesting the increased interaction between the two units. meso-Substitution with the N-butylcarbazole group on the BODIPY core rendered huge Stokes shifts (111–168 nm) and higher quantum yields as compared to meso-aryl BODIPY. An efficient energy transfer from the carbazole unit to the BODIPY core was observed by fluorescence spectroscopy for all the compounds 1–4. CV studies of compounds 1–4 showed anodic shifts of the reduction and oxidation potentials, suggesting that the meso-carbazole group is affecting the electronic properties of the BODIPY core and making them easier to reduce.

 Please wait while we load your content...
Please wait while we load your content...