Topological ferrimagnetic behaviours of coordination polymers containing manganese(ii) chains with mixed azide and carboxylate bridges and alternating F/AF/AF′/AF′/AF interactions†
Abstract
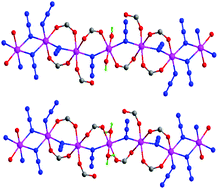
Two Mn(II) complexes with azide and a new zwitterionic tetracarboxylate ligand 1,2,4,5-tetrakis(4-carboxylatopyridinium-1-methylene)benzene (L1), {[Mn5(L1)2(N3)8(OH)2]·12H2O}n (1) and {[Mn5(L1)2(N3)8(H2O)2](ClO4)2·6H2O}n (2), have been synthesized and characterized crystallographically and magnetically. 1 and 2 contain similar alternating chains constructed by azide and carboxylate bridges. The independent sets of bridges alternate in an ABCCB sequence between adjacent Mn(II) ions: (EO-N3)2 double bridges (EO = end-on) (denoted as A), [(EO-N3)(OCO)2] triple bridges (denoted as B) and [(EO-N3)(OCO)] double bridges (denoted as C). The alternating chains are interlinked into 2D coordination networks by the tetrapyridinium spacers. Magnetic studies demonstrate that the magnetic coupling through the double EO azide bridges is ferromagnetic and that through mixed azide/carboxylate bridges is antiferromagnetic. The unprecedented F/AF/AF′/AF′/AF coupling sequence along the chain dictates an uncompensated ground spin state (S = 5/2 per Mn5 unit) and leads to one-dimensional topological ferrimagnetism, which features a minimum in the χT versus T plot.

 Please wait while we load your content...
Please wait while we load your content...