Hemilability of P(X)N-type ligands (X = O, N–H): rollover cyclometalation and benzene C–H activation from (P(X)N)PtMe2 complexes†‡
Abstract
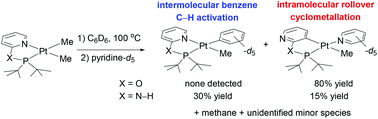
The thermolyses of (tBuP(O)N)PtMe2 (1, tBuP(O)N = (di-tert-butylphosphinito)pyridine) and (tBuP(N–H)N)PtMe2 (3, tBuP(N–H)N = (di-tert-butylphosphino)-2-aminopyridine) in benzene-d6 were investigated. With (tBuP(O)N)PtMe2, the product of a rollover cyclometalation of the pyridyl ring was observed in 80% yield along with formation of CH4. In contrast, thermolysis of (tBuP(N–H)N)PtMe2 resulted in competing rollover cyclometalation and intermolecular benzene C–H activation with production of a mixture of CH4 and CH3D.
- This article is part of the themed collection: The Carbon–Metal Bond and C–H Metalation

 Please wait while we load your content...
Please wait while we load your content...