4-Nitrophenyl- and 4′-nitro-1,1′-biphenyl-4-carboxylates attached to Mo2 quadruple bonds: ground versus excited state M2δ–ligand conjugation†
Abstract
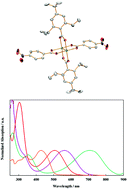
From the reactions between Mo2(TiPB)4, where TiPB = 2,4,6-triisopropylbenzoate and two equivalents of the carboxylic acid LH (LH = 4-nitrobenzoic acid and 4′-nitro[1,1′-biphenyl]-4-carboxylic acid) the compounds trans-M2(TiPB)2L2 have been prepared: I (L = 4-nitrobenzoate and M = Mo), II (L = 4′-nitro-1,1′-biphenylcarboxylate and M = Mo) and III (L = 4-nitrobenzoate and M2 = MoW). The compounds have been characterized by 1H NMR, UV-Vis and steady state emission spectroscopy, ns and fs transient absorption spectroscopy and cyclic voltammetry. These data are compared with predictions based on electronic structure calculations on model compounds where TiPB is substituted for formate. Together these data indicate stronger ground-state coupling of the Mo2δ and ligand π* systems in I relative to II but this order is reversed in the photo excited S11MLCT state. Attempts to prepare the W2 containing analogs were unsuccessful.

 Please wait while we load your content...
Please wait while we load your content...