Highly fluorescent complexes with 3-isocyanoperylene and N-(2,5-di-tert-butylphenyl)-9-isocyano-perylene-3,4-dicarboximide†
Abstract
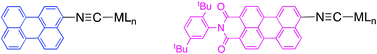
The perylene derivatives 3-isocyanoperylene (Per–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) (4a) and N-(2,5-di-tert-butylphenyl)-9-isocyano-perylene-3,4-dicarboximide (PMI–N
C) (4a) and N-(2,5-di-tert-butylphenyl)-9-isocyano-perylene-3,4-dicarboximide (PMI–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) (4b) were prepared and used to synthesize gold complexes [AuX(CNR)] (X = C6F5 (5a,b), C6F4-OnBu-p (6b)). The reaction of 5b and 6b with HNEt2 led to the carbene complexes [AuX{C(NEt2)(NHR)}] (7b, 8b), respectively. The molecular structure of complexes 7b and 8b have been determined by X-ray diffraction analysis showing intermolecular π-stacking of the perylene groups and C6F5 rings and no Au⋯Au interactions. The derivative compounds [M(CO)5(CNR)] (M = Cr (9a,b), Mo (10a,b) or W (11a,b)) and trans-[Pd(CNR)2(C6F3Cl2)2] (12a,b) were also prepared. All complexes exhibit fluorescence associated with the perylene fragment with emission quantum yields, in solution at room temperature, in the range 0.05–0.93 and emission lifetimes ∼ 4 ns. DFT calculations were performed of the absorption spectra of the ligands Per–N
C) (4b) were prepared and used to synthesize gold complexes [AuX(CNR)] (X = C6F5 (5a,b), C6F4-OnBu-p (6b)). The reaction of 5b and 6b with HNEt2 led to the carbene complexes [AuX{C(NEt2)(NHR)}] (7b, 8b), respectively. The molecular structure of complexes 7b and 8b have been determined by X-ray diffraction analysis showing intermolecular π-stacking of the perylene groups and C6F5 rings and no Au⋯Au interactions. The derivative compounds [M(CO)5(CNR)] (M = Cr (9a,b), Mo (10a,b) or W (11a,b)) and trans-[Pd(CNR)2(C6F3Cl2)2] (12a,b) were also prepared. All complexes exhibit fluorescence associated with the perylene fragment with emission quantum yields, in solution at room temperature, in the range 0.05–0.93 and emission lifetimes ∼ 4 ns. DFT calculations were performed of the absorption spectra of the ligands Per–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C and PMI–N
C and PMI–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C and representative complexes [Au(C6F5)(CNR)], [Cr(CO)5(CNR)], showing a perylene-dominated intraligand π–π* emissive state, from the HOMO and LUMO orbitals of the perylene chromophore, but with significantly different absorption maxima by the influence of the metal fragment, particularly significant in the Per–N
C and representative complexes [Au(C6F5)(CNR)], [Cr(CO)5(CNR)], showing a perylene-dominated intraligand π–π* emissive state, from the HOMO and LUMO orbitals of the perylene chromophore, but with significantly different absorption maxima by the influence of the metal fragment, particularly significant in the Per–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C derivatives.
C derivatives.

 Please wait while we load your content...
Please wait while we load your content...