Construction of covalent- and hydrogen-bonded assemblies from 1′,1′′′-biferrocenediboronic acid as a new organobimetallic building block†
Abstract
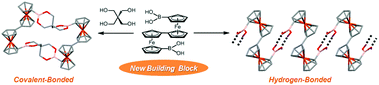
1′,1′′′-Biferrocenediboronic acid (1) was synthesized from 1′,1′′′-dibromobiferrocene by a typical procedure of converting Br to B(OH)2 groups in 76% yield and identified by 1H-, 13C- and 11B-NMR and ESI-MS. X-ray diffraction (XRD) studies showed that, in non-solvated crystals (Form I), the new organobimetallic building block 1 formed 1D hydrogen-bonded networks (i.e., chain) with octaatomic rings composed of the neighbouring two molecules. In solvated crystals with a composition of (1)3(THF)2 (Form II), 1 exists in two conformers (Conformers A and B) with respect to the rotation of the CpB(OH)2 moieties relative to the Cp rings of the fulvalenide moieties; Conformer A formed 1D hydrogen-bonded networks laterally hydrogen-bonding with THF molecules while Conformer B formed a new planar hydrogen-bonded motif involving four B(OH)2 groups and stepwise laminated networks of the planar motif. A macrocyclic tetraferrocenyl boronate ester 2 was synthesized by cyclocondensation between 1 and pentaerythritol in 33% yield and identified by 1H-, 13C- and 11B-NMR, ESI-MS and XRD. In electrochemical measurements, the cyclocondensed compound 2 exhibited four defined reversible waves with a total spread of 756 mV in CH2Cl2 containing n-Bu4NBArF4 (ArF = 3,5-bis(trifluoromethyl)phenyl), displaying both intra- and inter-biferrocenyl interactions.

 Please wait while we load your content...
Please wait while we load your content...