Hydrosilylation of aldehydes and ketones catalyzed by hydrido iron complexes bearing imine ligands†
Abstract
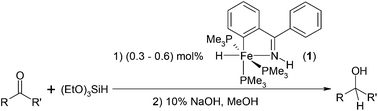
Two new hydrido iron complexes (2 and 4) were synthesized by the reactions of (4-methoxyphenyl)phenylketimine ((4-MeOPh)PhC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH) with Fe(PMe3)4 or FeMe2(PMe3)4. The molecular structures of complexes 2 and 4 were confirmed by X-ray single crystal diffraction. Using hydrido iron complexes (1–4) as catalysts, the hydrosilylations of aldehydes and ketones were investigated. The four complexes were effective catalysts for this reduction reaction. Complex 1 among them is the best catalyst.
NH) with Fe(PMe3)4 or FeMe2(PMe3)4. The molecular structures of complexes 2 and 4 were confirmed by X-ray single crystal diffraction. Using hydrido iron complexes (1–4) as catalysts, the hydrosilylations of aldehydes and ketones were investigated. The four complexes were effective catalysts for this reduction reaction. Complex 1 among them is the best catalyst.

 Please wait while we load your content...
Please wait while we load your content...