Crown ether adducts of light alkali metal triphenylsilyls: synthesis, structure and hydrosilylation catalysis†
Abstract
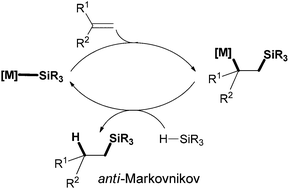
Alkali metal triphenylsilyls [Li(12-crown-4)SiPh3]·(thf)0.5 (2), [Na(15-crown-5)SiPh3]·(thf)0.5 (3) and [K(18-crown-6)SiPh3(thf)] (4) were synthesized using 1,1,1-trimethyl-2,2,2-triphenyldisilane (Ph3SiSiMe3) and isolated in high yields. Solid state structures were determined by single crystal X-ray diffraction. These alkali metal silyls catalyzed the regioselective hydrosilylation of 1,1-diphenylethylene to give the anti-Markovnikov product. The presence of crown ethers enhanced the reactivity of the metal silyls in hydrosilylation catalysis.
- This article is part of the themed collection: New Expeditions in Polar Organometallic Chemistry

 Please wait while we load your content...
Please wait while we load your content...