Three Mn(ii) coordination polymers with a bispyridyl-based quinolinate ligand: the anion-controlled tunable structural and magnetic properties†
Abstract
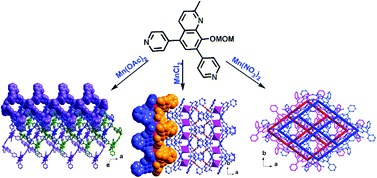
Three new Mn(II) coordination polymers, namely [Mn3L6·2H2O] (1), [MnL2] (2), and [MnL2·2H2O] (3), were prepared by solvothermal reactions of Mn(II) salts with a bispyridyl-based quinolinate ligand. All complexes were characterized by elemental analysis, IR spectra, powder and single-crystal X-ray crystallography. Single crystal X-ray studies show that these coordination polymers exhibit a structural diversification due to the different counteranions (OAc−, Cl−, and NO3−). Complex 1 has a 2D supramolecular structure with a cyclic tetramer Mn3L6 secondary building unit. Complex 2 possesses a rhombohedral grid network containing a type of meso-helical chain (P + M) constructed via the metal–ligand coordination interaction. Complex 3 features a 3D non-porous structure based on the arrangement of 2D grids. Magnetic susceptibility measurements indicate that the three Mn(II) polymers 1–3 show disparate magnetic properties due to their different supramolecular structures.

 Please wait while we load your content...
Please wait while we load your content...