Heterodinuclear MII–LnIII single molecule magnets constructed from exchange-coupled single ion magnets†
Abstract
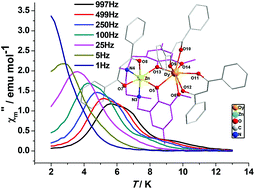
The synthesis and characterization of four dinuclear 3d–4f complexes [MIILnIII(L)(DBM)3] (ZnDy = 1, CoY = 2, CoDy = 3·3.5CH3CN, CoGd = 4·3.5CH3CN) are reported (H2L = N,N′-dimethyl-N,N′-(2-hydroxy-3-methoxy-5-methyl-benzyl)ethylenediamine, DBM− = anion of 1,3-diphenyl-propane-1,3-dione). In each of the four complexes, the MII ion occupies the internal N2O2 site whereas the LnIII ion occupies the external O4 site. There are diphenoxo bridges between the MII and LnIII ions in these complexes. The remaining coordination sites are occupied by three DBM− anions. Direct current (dc) magnetic susceptibility measurements indicate the presence of intramolecular ferromagnetic interactions in complexes 3 and 4. The magnetic coupling constant, JCoGd, of complex 4 is estimated to be 0.26 cm−1 (H = −2JCoGdSCoSGd). Alternating current (ac) magnetic susceptibility studies reveal that complexes 1 and 2 show field-induced single molecule magnet behavior, with ΔE values of 36.5 K and 8.56 K, respectively. Complex 3 shows frequency dependent out-of-phase signals, indicating the presence of a slow relaxation of the magnetization, whereas complex 4 does not display slow magnetization relaxation.

 Please wait while we load your content...
Please wait while we load your content...