Di-, tri- and tetranuclear molecular vanadium phosphonates: a chloride encapsulated tetranuclear bowl†
Abstract
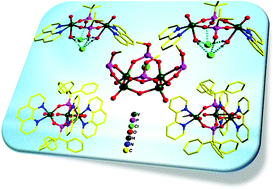
The reaction of vanadium(III) trichloride with tert-butylphosphonic acid (t-BuPO3H2) in the presence of 1,10-phenanthroline/2,2′-bipyridine as an ancillary ligand in acetonitrile at room temperature afforded two dinuclear dicationic vanadium(IV) complexes [(VO)2(phen)2{t-BuPO2(OH)}2(OH2)2]·2Cl [1] and [(VO)2(bipy)2{t-BuPO2(OH)}2(OH2)2]·2Cl [2]. On the other hand, when the reaction was carried out in methanol, the dinuclear vanadium(V) complex [(VO)2(bipy)2(μ2-O)2(t-BuPO3)2]·2CH3OH·0.5CH2Cl2 [3] was isolated. While 1 and 2 contain two six-membered V2P2O4 rings, 3 contains a unique four-membered V2O2 ring. Replacement of tert-butylphosphonic acid by tritylphosphonic acid (Ph3CPO3H2) under the same reaction conditions in methanol leads to the formation of dicationic trinuclear vanadium(IV) complexes [(VO)3(phen)3(Ph3CPO3)2(OH2)3]·CHCl3·2(OH)·2MeOH·1.5H2O [4] and [(VO)3(bipy)3(Ph3CPO3)2(CH3OH)3]·2(OH)·4CH3OH·5H2O [5]. In these complexes, the triangular V(IV) platform is held together by two bicapping tripodal phosphonate ligands. Replacement of the chelating 2,2′-bipyridine ligand with 3,5-dimethyl-1H-pyrazole, under the same reaction conditions, afforded a tetranuclear vanadium(V) complex [{(VO)4(Ph3CPO3Me)4(μ-O)4}Cl]{3,5-Me2PzH2}·3C7H8·H2O·CH3OH [6]. Remarkably 6 possesses a unique bowl-shaped structure encapsulating a chloride anion.

 Please wait while we load your content...
Please wait while we load your content...