Possible intermediates of Cu(phen)-catalyzed C–O cross-coupling of phenol with an aryl bromide by in situ ESI-MS and EPR studies†
Abstract
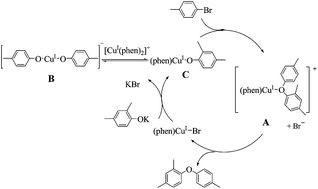
The C–O coupling reaction between 2,4-dimethylphenol and 4-bromotoluene catalyzed by the CuI/K2CO3/phen system can be inhibited by the radical scavenger cumene. Complexes [Cu(I)(phen)(1-(2,4-dimethylphenoxy)-4-methylbenzene)]+ (denoted as A), {H[Cu(I)(phen)(2,4-dimethylphenoxy)]}+ and [Cu(I)(2,4-dimethylphenoxy)2]− (denoted as B) were observed by in situ electrospray ionization mass spectrometry (ESI-MS) analysis of the copper(I)-catalyzed C–O coupling reaction under the catalytic reaction conditions indicating that they could be intermediates in the reaction. The in situ EPR study of the reaction solution detected the Cu(II) species with a fitted g value of 2.188. A catalytic cycle with a single electron transfer (SET) step was proposed based on these observations.

 Please wait while we load your content...
Please wait while we load your content...