Strong Lewis acid air-stable cationic titanocene perfluoroalkyl(aryl)sulfonate complexes as highly efficient and recyclable catalysts for C–C bond forming reactions†
Abstract
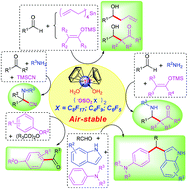
A series of strong Lewis acid air-stable titanocene perfluoroalkyl(aryl)sulfonate complexes Cp2Ti(OH2)2(OSO2X)2·THF (X = C8F17, 1·THF; X = C4F9, 2·H2O·THF; X = C6F5, 3) were successfully synthesized by the treatment of Cp2TiCl2 with C8F17SO3Ag, C4F9SO3Ag and C6F5SO3Ag, respectively. In contrast to well-known titanocene bis(triflate), these complexes showed no change in open air over three months. TG-DSC analysis showed that 1·THF, 2·H2O·THF and 3 were thermally stable at 230 °C, 220 °C and 280 °C, respectively. Conductivity measurements showed that these complexes underwent ionic dissociation in CH3CN solution. X-ray analysis results confirmed that 2·H2O·THF and 3 were cationic. ESR spectra showed that the Lewis acidity of 1·THF (1.06 eV) was higher than that of Sc3+ (1.00 eV) and Y3+ (0.85 eV). UV/Vis spectra showed a significant red shift due to the strong complex formation between 10-methylacridone and 2·H2O·THF. Fluorescence spectra showed that the Lewis acidity of 2 (λem = 477 nm) was higher than that of Sc3+ (λem = 474 nm). These complexes showed high catalytic ability in various carbon–carbon bond forming reactions. Moreover, they show good reusability. Compared with 1·THF, 2·H2O·THF and 3 exhibit higher solubility and better catalytic activity, and will find broad applications in organic synthesis.

 Please wait while we load your content...
Please wait while we load your content...