Copper(ii) complexes supported by click generated mixed NN, NO, and NS 1,2,3-triazole based ligands and their catalytic activity in azide–alkyne cycloaddition†
Abstract
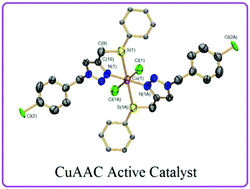
The preparation and characterization of four new copper(II) complexes supported by click generated mixed NN, NO, and NS 1,2,3-triazoles are reported. The four complexes display a 1 : 2 copper/ligand ratio and give monomeric units in the solid state. Crystal structures demonstrate that depending on the flexibility of the ligand NX (X = O, N, S) pendant arm, the coordination environment around the metal center can feature square planar or octahedral geometries. All four complexes are catalytically active at room temperature in a copper-catalyzed alkyne–azide cycloaddition (CuAAC) reaction using sodium ascorbate as a reducing agent and water–ethanol as a solvent mixture. Complex 8 supported by the NS ligand displayed the best catalytic performance of the series allowing for the easy and high yielding preparation of a variety of mono-, bis- and tris-1,2,3-triazoles under low catalyst loadings.

 Please wait while we load your content...
Please wait while we load your content...