The “innocent” role of Sc3+ on a non-heme Fe catalyst in an O2 environment†
Abstract
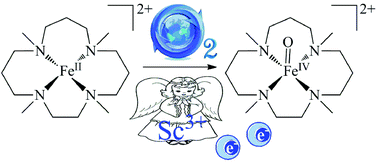
Density functional theory calculations have been used to investigate the reaction mechanism proposed for the formation of an oxoiron(IV) complex [FeIV(TMC)O]2+ (P) (TMC = 1,4,8,11-tetramethylcyclam) starting from a non-heme reactant complex [FeII(TMC)]2+ (R) and O2 in the presence of acid H+ and reductant BPh4−. We also addressed the possible role of redox-inactive Sc3+ as a replacement for H+ acid in this reaction to trigger the formation of P. Our computational results substantially confirm the proposed mechanism and, more importantly, support that Sc3+ could trigger the O2 activation, mainly dictated by the availability of two electrons from BPh4−, by forming a thermodynamically stable Sc3+–peroxo–Fe3+ core that facilitates O–O bond cleavage to generate P by reducing the energy barrier. These insights may pave the way to improve the catalytic reactivity of metal–oxo complexes in O2 activation at non-heme centers.
- This article is part of the themed collection: Synergy between Experiment and Theory

 Please wait while we load your content...
Please wait while we load your content...