Reduction of an Fe(i) mesityl complex induced by π-acid ligands†
Abstract
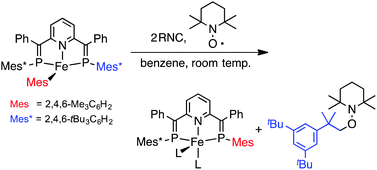
Treatment of the Fe(I) mesityl complex [Fe(Mes)(BPEP-Ph)] (BPEP-Ph = 2,6-bis[1-phenyl-2-(2,4,6-tri-tert-butylphenyl)-2-phosphaethenyl]pyridine) with π-acid ligands (L = CO, RNC) leads to one-electron reduction via Mes group migration from Fe to P, followed by homolytic elimination of the 2,4,6-tBu3C6H2 group, to afford Fe(0) complexes of the formula [Fe(L)2(BPEP-Ph*)] (BPEP-Ph* = 2-[1-phenyl-2-mesityl-2-phosphaethenyl]-6-[1-phenyl-2-(2,4,6-tri-tert-butylphenyl)-2-phosphaethenyl]pyridine). This reduction process is supported by radical trapping experiments and theoretical studies. The 2,4,6-tBu3C6H2˙ radical is captured by 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) in high yield. DFT calculations reveal the mechanism of Mes group migration with a reasonable energy profile.

 Please wait while we load your content...
Please wait while we load your content...