Facile synthesis of highly fluorescent BF2 complexes bearing isoindolin-1-one ligand†
Abstract
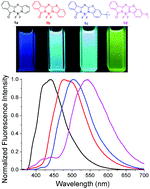
Stable organoboron complexes as classic fluorescent molecules have found various applications in biotechnology and material sciences. A new class of isoindolin-1-one based BF2 complexes containing pyridine or benzothiazole groups has been prepared from a facile “one-pot” reaction and characterized structurally, spectroscopically and electrochemically. These novel dyes show broad and intense absorption and emission bands in solution with high fluorescence quantum yields. Significantly larger Stokes shifts and higher photostability were observed for these dyes in comparison to those of classical BODIPYs.

 Please wait while we load your content...
Please wait while we load your content...