Molecular and polymeric zinc(ii) phosphonates: isolation of an octanuclear ellipsoidal ensemble†
Abstract
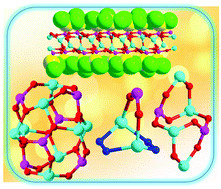
The reaction of zinc(II) perchlorate with trichloromethyl phosphonic acid at room temperature afforded, upon crystallization, a two-dimensional layered coordination polymer possessing a dinuclear repeat unit, [{Zn2(Cl3CPO3)2(H2O)3}·1.5H2O]n (1). Modification of the above reaction by involving a co-ligand afforded the tetranuclear complex, [{Zn4(η1-DMPzH)6(Cl3C–PO3)2}(μ-OH)2(ClO4)2] (2). The molecular structure of 2 reveals that the tetranuclear core is non-planar and consists of three contiguous inorganic rings which include one 8-membered Zn2P2O4 ring and two six-membered Zn2PO3 rings. Replacement of Zn(ClO4)2·6H2O with ZnCl2 under the same reaction conditions that afforded 2 allowed the formation of the dinuclear complex [{(ZnCl)2(η2-Pz)2(Cl3CPO3)}(Et3NH)2] (3). 3 possesses a bicyclic core containing a seven-membered Zn2N2O2P ring. In 3, the phosphoryl oxygen atom (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) is involved in a bifurcated hydrogen bonding interaction with the triethylammonium cation. The reaction of ZnCl2 and 2,3,5,6-(Me)4C6HCH2PO3H2 afforded the octanuclear complex [Zn8(Cl)6{2,3,5,6-(Me)4C6HCH2PO3}6(Et3N)2](Et3NH)2]·2n-hexane·3H2O (4). The core of 4 is ellipsoid-shaped with the end–end polar distance (C–C) being ∼20 Å.
O) is involved in a bifurcated hydrogen bonding interaction with the triethylammonium cation. The reaction of ZnCl2 and 2,3,5,6-(Me)4C6HCH2PO3H2 afforded the octanuclear complex [Zn8(Cl)6{2,3,5,6-(Me)4C6HCH2PO3}6(Et3N)2](Et3NH)2]·2n-hexane·3H2O (4). The core of 4 is ellipsoid-shaped with the end–end polar distance (C–C) being ∼20 Å.

 Please wait while we load your content...
Please wait while we load your content...