Effects of crystalline phase and morphology on the visible light photocatalytic H2-production activity of CdS nanocrystals
Abstract
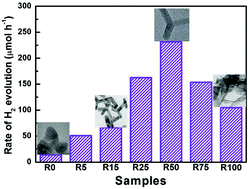
Visible light photocatalytic H2-production from aqueous solutions is of great importance for its potential application in converting solar energy into chemical energy. In this study, a series of CdS nanostructures with different contents of wurtzite (WZ) and zinc blende (ZB) phases were successfully synthesized by a simple solvothermal route in an ethylenediamine and ethylene glycol mixed solution. The solvent volume ratio of ethylenediamine in the mixed solution (R) exhibited an obvious influence on the crystalline phase and morphology of the resulting CdS products. With increasing R, the percentage of wurtzite first increased and then decreased, whilst the morphology changed from nanoparticles to multi-armed nanorods, and finally to long rods and sheets. The prepared multi-armed CdS nanorod samples showed especially high and stable photocatalytic H2-production activity with Pt (0.25 wt%) as a co-catalyst and lactic acid aqueous solution as a sacrificial reagent under visible light irradiation. The optimized CdS nanorods with the highest percentage (64%) of the WZ phase exhibited a high H2-production rate of 231.4 μmol h−1 (about 16.6 times higher than that of CdS nanoparticles with a low percentage (38.4%) of WZ CdS) and with a quantum efficiency (QE) of 28% at 420 nm. This high photocatalytic H2-production activity could be attributed to the results of the positive synergistic effects of the hexagonal WZ phase and morphology of multi-armed nanorods.

 Please wait while we load your content...
Please wait while we load your content...