Variable photon energy photoelectron spectroscopy of tris-cyclopentadienyl lanthanides
Abstract
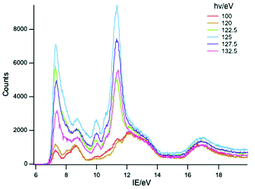
The gas phase photoelectron (PE) spectra of LnCp3 (Cp = η-C5H5; Ln = Pr, Nd, Sm), measured with a wide range of photon energy, are reported. Resonances observed in the photon energy regions of 4d to 4f excitation enable identification of ion states resulting from 4f ionization. For all three compounds molecular ion states characteristic of both 4fn and 4fn−1 configurations are observed (Pr, n = 2; Nd, n = 3; Sm, n = 6). The molecular ion ground states have a hole in the uppermost ligand orbital of a′ symmetry and are reached by either ligand or f electron ionization. The results are discussed in the context of the previously reported spectra of the Ce, Yb and Lu analogues. For YbCp3 f orbital/ligand interaction is proposed in the molecular ground state and for CeCp3+ in the molecular ion ground state. For PrCp3 and NdCp3 final state effects are proposed as the origin of the dual configuration structure in their PE spectra. When the contributing orbitals are close in energy the 4f/a′ interaction can give rise to significant covalent bonding even in the absence of effective overlap.

 Please wait while we load your content...
Please wait while we load your content...