Structure and hydrogen bonding of the hydrated selenite and selenate ions in aqueous solution†
Abstract
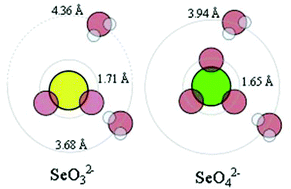
The structure and hydrogen bonding of the hydrated selenite, SeO32−, and selenate, SeO42−, ions have been studied in aqueous solution by large angle X-ray scattering (LAXS), EXAFS and double difference infrared (DDIR) spectroscopy. The mean Se–O bond distances are 1.709(2) and 1.657(2) Å, respectively, as determined by LAXS, and 1.701(3) and 1.643(3) Å by EXAFS. These bond distances are slightly longer than the mean distances found in the solid state, 1.691 and 1.634 Å, respectively. The structures of HSeO3−, H2SeO3 and HSeO4− in aqueous solution have been determined by EXAFS giving the same Se–O bond distances as for the selenite and selenate ions, respectively. The mean Se⋯Ow distance to the water molecules hydrogen binding to selenite oxygens is 3.87(2) Å, and it is 4.36(8) Å to those clustered outside the lone electron-pair. The selenate ion has a symmetric hydration shell with only one Se⋯Ow distance, 3.94(2) Å. The mean Se–O⋯Ow angle in the hydrated selenite ion is 114.5°, and the large temperature factor of the Se⋯Ow distance strongly indicates equilibrium between two and three water molecules hydrogen bound to the selenite oxygens. The mean Se–O⋯Ow angle in the hydrated selenate ion is 120° which strongly indicates that two water molecules hydrogen bind to the selenate oxygens. The DDIR spectra show peaks for the affected water bound to the selenite and selenate ions at 2491 ± 2 and 2480 ± 39 cm−1, respectively, compared to 2509 cm−1 in pure water. This shows that the selenite and selenate ions shall be regarded as weak structure makers.

 Please wait while we load your content...
Please wait while we load your content...