Lanthanide ion exchange properties of a coordination polymer consisting of di(2-ethylhexyl) phosphoric acid and trivalent metal ions (Ce3+, Fe3+, or Al3+)†
Abstract
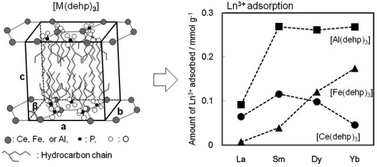
Three kinds of coordination polymers ([M(dehp)3], M = Ce, Fe, or Al) were prepared by mixing the sodium form (Na(dehp)) of di(2-ethylhexyl) phosphoric acid and MCl3 in an ethanol–water binary mixture. They have monoclinic crystalline structure with similar lattice parameters. The lanthanide ion (Ln3+ = La3+, Sm3+, Dy3+, or Yb3+) exchange properties were studied in a 20 : 80 vol% ethanol–water binary mixture containing 2 mM Ln(NO3)3 at room temperature. The rate of Ln3+ adsorption is relatively slow; it requires over 3 weeks to reach equilibrium. [M(dehp)3] has different Ln3+ affinities depending on the kind of central metal ions: the affinity order at 3 week adsorption is Yb3+ < La3+ < Dy3+ < Sm3+ for [Ce(dehp)3], La3+ < Sm3+ < Dy3+ < Yb3+ for [Fe(dehp)3], and La3+ < Sm3+, Dy3+, Yb3+ for [Al(dehp)3]. The difference in affinity order can be explained by two factors: the coordination preference and steric strain caused by the polymeric structure. The chemical and structural analyses suggested that the Ln3+ adsorption progresses first by the central M3+/Ln3+ exchange, followed by a morphological change to a rod-like or fibrous form by a solid phase reaction. In the case of [Fe(dehp)3], the eluted Fe3+ may be hydrolyzed and precipitated as amorphous iron hydroxide.

 Please wait while we load your content...
Please wait while we load your content...