The influence of H-bonding on the ‘ambidentate’ coordination behaviour of the thiocyanate ion to Cd(ii): a combined experimental and theoretical study†
Abstract
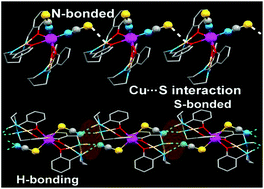
Two new trinuclear hetero-metallic copper(II)–cadmium(II) complexes [(CuL)2Cd(NCS)2] (1) and [(CuLR)2Cd(SCN)2] (2) have been synthesized using [CuL] and [CuLR] as “metalloligands” (where H2L = N,N′-bis(salicylidene)-1,4-butanediamine and H2LR = N,N′-bis(2-hydroxybenzyl)-1,4-butanediamine) respectively. Both the complexes were characterized by elemental analysis, various spectroscopic methods and single crystal XRD. Complex 1 is an angular trinuclear species, in which two terminal four-coordinate square planar “metalloligands” [CuL] are coordinated to a central Cd(II) through double phenoxido bridges along with two mutually cis nitrogen atoms of terminal thiocyanate ions. In complex 2, which is linear, in addition to the double phenoxido bridge, two SCN− coordinate to the trans positions of the central octahedral Cd(II) via S atoms. Theoretical calculations on the energetic difference between the two possible coordination modes of the thiocyanate anion to the Cd atom reveal that N-coordination is preferred over S-coordination in agreement with the much greater abundance of the reported N-bonded structures. In 2, there is a strong N–H⋯NCS–Cd H-bonding interaction, the binding energy of which is computed to be approximately −9.3 kcal mol−1, which is sufficient to compensate the 9.0 kcal mol−1 of energetic cost due to the unusual Cd–SCN coordination mode.

 Please wait while we load your content...
Please wait while we load your content...