Role of different platinum precursors on the formation and reaction mechanism of FePt nanoparticles and their electrocatalytic performance towards methanol oxidation†
Abstract
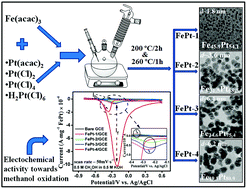
We report the formation mechanism of FePt nanoparticles (NPs) by a high temperature polyol method using an equimolar ratio of Fe and Pt-precursor with different Pt-precursors. Pt(acac)2, PtCl2, PtCl4 and H2PtCl6·H2O were used as Pt-precursors and Fe(acac)3 as the only Fe-precursor. Different stoichiometric compositions along with variation in size were obtained by using different precursors of Pt. Nearly, equiatomic FePt having a size ∼2 nm was formed with Pt(acac)2. However, Pt rich phases were formed using all other precursors with a size ranging between 3.6 to 6.4 nm. It was found that the atomic percentage (at%) of Fe in the FePt NPs depends on the reaction parameters. The decomposition behaviour of Fe and Pt-precursors were examined by vibrating sample magnetometer and thermogravimetric measurements. A possible reaction mechanism for Fe depleted FePt formation is proposed which suggests that the reduction potential and decomposition behaviour of the organic and inorganic salts of Pt significantly modify the nucleation behaviour. The electrocatalytic properties of all the four nanomaterials towards methanol oxidation have been investigated by cyclic voltammetry. It is found that Fe19Pt81 with an average size of 6.2 nm shows the highest catalytic response.

 Please wait while we load your content...
Please wait while we load your content...