Donor functionalized ruthenium N-heterocyclic carbene complexes in alcohol oxidation reactions†
Abstract
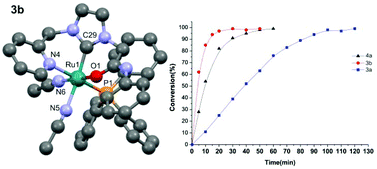
N-Pyridyl, N′-amido functionalized imidazolium bromides were obtained in high yields as an N-heterocyclic carbene (NHC) precursor and used as bidentate or a pincer ligands to obtain ruthenium complexes via a silver NHC transmetallation route. The incorporation of a phenyl group as an amido-N substituent (R = Ph) results in a bidentate coordination mode through the CNHC and Npyridyl donors, whereas in its absence (R = H) a pincer coordination mode was observed through the Npyridyl^CNHC^Oamido donors. The ruthenium complex featuring a pincer type NCO coordination mode with a protic NH function adjacent to the coordinating Oamido atom was found to efficiently catalyse the oxidation of activated alcohols effecting quantitative conversions within 30 minutes. However the oxidation of deactivated alcohols required longer reaction times to effect the quantitative transformation.

 Please wait while we load your content...
Please wait while we load your content...