Binuclear ruthenium η6-arene complexes with tetradentate N,S-ligands containing the ortho-aminothiophenol motif†
Abstract
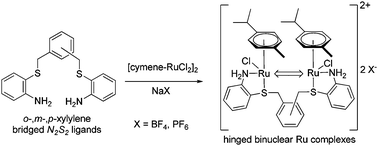
A series of cationic binuclear (η6-cymene–Ru)2 complexes with N2S2-ligands were synthesized in 64% to 85% yield by reaction of [Ru(η6-cymene)Cl2]2 with bis-S,S′-(ortho-aminothiophenol)-xylenes as BF4− and PF6− salts. The compounds were studied using NMR, HRMS, UV-vis and IR spectroscopy, EA and inductively coupled plasma (ICP) MS. It was determined that the hinged binuclear Ru complexes were anti and syn diastereomers obtained in 2 : 1 ratio for ortho- and meta-xylylene bridged ligands and in a 1 : 1 ratio for the para-xylylene bridged ligand. An anion effect was found for the presence of NaBF4 with the meta-xylylene bridged system yielding the targeted binuclear Ru complex and a mononuclear Ru complex. This mononuclear S,S′-coordinated η6-cymene Ru chloride structure lacked amine–metal coordination and was obtained in a 1 : 3 ratio of anti : syn diastereomers which were insoluble in CH2Cl2 and soluble in DMSO and DMF. X-ray crystallographic analysis was obtained for the N2S2 ligand, 1,2-bis{(2-aminophenyl)thiomethyl}benzene, showing a CS symmetry with amine groups facing outwards with a tilt of 28.95° from the ortho-aminothiophenol pendant ring. The interatomic sulfur–sulfur distance (S–S′) is 4.6405 Å within the crystal structure while accommodating a potential metal bite angle from 1.0 Å to 5.9 Å when allowing rotation of the methylene phenyl bond.

 Please wait while we load your content...
Please wait while we load your content...