Synthesis and catalytic alcohol oxidation and ketone transfer hydrogenation activity of donor-functionalized mesoionic triazolylidene ruthenium(ii) complexes†
Abstract
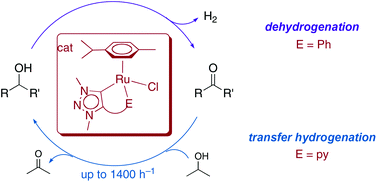
We report on the synthesis of a variety of C,E-bidentate triazolylidene ruthenium complexes that comprise different donor substituents E (E = C: phenyl anion; E = O: carboxylate, alkoxide; E = N: pyridine at heterocyclic carbon or nitrogen). Introduction of these donor functionalities is greatly facilitated by the synthetic versatility of triazoles, and their facile preparation routes. Five different complexes featuring a C,E-coordinated ruthenium center with chloride/cymene spectator ligands and three analogous solvento complexes with MeCN spectator ligands were prepared and evaluated as catalyst precursors for direct base- and oxidant-free alcohol dehydrogenation, and for transfer hydrogenation using basic iPrOH as a source of dihydrogen. In both catalytic reactions, the neutral/mono-cationic complexes with chloride/cymene spectator ligands performed better than the solvento ruthenium complexes. The donor functionality had a further profound impact on catalytic activity. For alcohol dehydrogenation, the C,C-bidentate phenyl-triazolylidene ligand induced highest conversions, while carboxylate or pyridine donor sites gave only moderate activity or none at all. In contrast, transfer hydrogenation is most efficient when a pyridyl donor group is linked to the triazolylidene via the heterocyclic carbon atom, providing turnover frequencies as high as 1400 h−1 for cyclohexanone transfer hydrogenation. The role of the donor group is discussed in mechanistic terms.
- This article is part of the themed collection: New Talent: Europe

 Please wait while we load your content...
Please wait while we load your content...