Carbene formation upon reactive dissolution of metal oxides in imidazolium ionic liquids†
Abstract
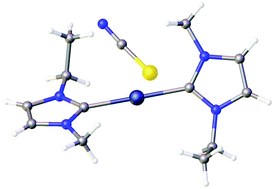
Metal oxides were found to dissolve in different imidazolium ionic liquids with a hydrogen atom in the C2 position of the imidazolium ring, but not if a methyl substituent was present in the C2 position. The crystal structure of the product that crystallised from an ionic liquid containing dissolved silver(I) oxide showed that this was a silver(I) carbene complex. The presence of carbenes in solution was proven by 13C NMR spectroscopy and the reactions were also monitored by Raman spectroscopy. The dissolution of other metal oxides, namely copper(II) oxide, zinc(II) oxide and nickel(II) oxide, was also studied in imidazolium ionic liquids and it was found that stable zinc(II) carbenes were formed in solution, but these did not crystallise under the given experimental conditions. A crystalline nickel(II) carbene complex could be obtained from a solution of nickel(II) chloride dissolved in a mixture of 1-butyl-3-methylimidazolium and 1-ethyl-3-methylimidazolium acetate.

 Please wait while we load your content...
Please wait while we load your content...