C–Cl bond activation and catalytic hydrodechlorination of hexachlorobenzene by cobalt and nickel complexes with sodium formate as a reducing agent†
Abstract
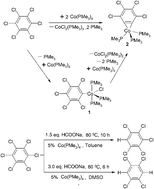
A benzyne cobalt complex, Co(η2-C6Cl4)(PMe3)3 (2), was generated from the reaction of hexachlorobenzene with 2 equiv. of Co(PMe3)4 through selective activation of two C–Cl bonds of hexachlorobenzene. Meanwhile, the byproduct CoCl2(PMe3)3 was also confirmed by IR spectra. The cobalt(II) complex, CoCl(C6Cl5)(PMe3)3 (1), as an intermediate in the formation of aryne complex 2, was also isolated by the reaction of hexachlorobenzene with the stoichiometric amount of Co(PMe3)4. Complex 2 could be obtained by the reaction of 1 with Co(PMe3)4. Under similar reaction conditions, the reaction of Ni(PMe3)4 with hexachlorobenzene afforded only a mono-(C–Cl) bond activation nickel(II) complex, NiCl(C6H5)(PMe3)2 (5). The expected benzyne nickel complex was not formed. The structures of complexes 2 and 5 were determined by X-ray single crystal diffraction. Successful selective hydrodechlorinations of hexachlorobenzene were studied and in the presence of Co(PMe3)4 or Ni(PMe3)4 as catalysts and sodium formate as a reducing agent pentachlorobenzene and 1,2,4,5-tetrachlorobenzene were obtained. The catalytic hydrodechlorination mechanism is proposed and discussed.

 Please wait while we load your content...
Please wait while we load your content...