Homoleptic aminophenolates of Zn, Mg and Ca. Synthesis, structure, DFT studies and polymerization activity in ROP of lactides†
Abstract
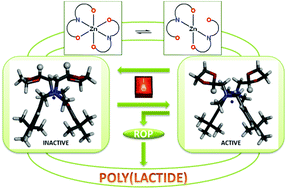
The reaction of MgBu2, ZnEt2 or Ca(OiPr)2 with 2 eq. of three-coordinating N-[methyl(2-hydroxy-3,5-dimethylphenyl)]-N-methyl-N-methyl-1,3-oxolaneamine (mpoa-H) or N-[methyl(2-hydroxy-3,5-di-tert-butylphenyl)]-N-methyl-N-methyl-1,3-oxolaneamine (tbpoa-H) gave neutral, monomeric [Mg(mpoa)2], [Zn(mpoa)2], [Zn(tbpoa)2], and [Ca(tbpoa)2] as white powders in 58–90% yields. The resulting aminophenolates were characterized in solution by NMR showing, in the case of [Zn(tbpoa)2], interesting dynamics. [Zn(tbpoa)2] and [Ca(tbpoa)2] were characterized by X-ray crystallography to show the Zn atom to be pseudo-octahedrally coordinated and the Ca atom in six-coordination mode. The new homoleptic complexes were tested in the polymerization of lactide with an external alcohol to reveal stable behaviour (during the polymerization process) only in the case of [Zn(tbpoa)2]. The high activity of the catalyst was correlated with a ligand flexibility that was further supported by theoretical studies.

 Please wait while we load your content...
Please wait while we load your content...