Base-mediated transformation of the agostic (π-allyl)-closo-rhodacarboranes into complexes with an open (1-5-η5)-pentadienyl ligand†
Abstract
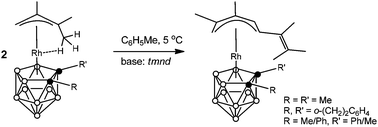
The reactions of agostic (CH3⋯Rh) complexes [3-{(1-3-η3)-1,1,2-trimethylallyl}-1-R-2-R′-closo-3,1,2-RhC2B9H9] [1: R = R′ = Me; 2: R,R′ = μ-(ortho-xylylene); 3: R = Ph, R′ = Me] in a cooled (+5 °C) toluene solution with the strong non-nucleophilic base N,N,N′,N′-tetramethylnaphthalene-1,8-diamine (tmnd) formally involve a linear coupling of the π-allyl ligand of one molecule of the complex to the π-allyl ligand of the second molecule of the complex, ultimately forming the structurally novel mononuclear species [3-{(1-5-η5)-2,3-dimethyl-5-(3-methylbuten-2-yl)pentadienyl}-1-R-2-R′-closo-3,1,2-RhC2B9H9] (4, 5) and (6a,b, a mixture of diastereomers). Complexes 5 and 6a,b were also prepared using weaker bases such as PPh3 or even EtOH instead of tmnd. All new complexes 4–6 were characterized by a combination of analytical and multinuclear NMR data, as well as by single-crystal X-ray diffraction studies of two selected species 5 and the major diastereomer of 6, which revealed an open η5-pentadienyl coordination mode of the hydrocarbon ligands in these complexes.
- This article is part of the themed collection: Carboranes

 Please wait while we load your content...
Please wait while we load your content...