A lanthanum chelate possessing an open-channel framework with water nanotubes: properties and desalination†
Abstract
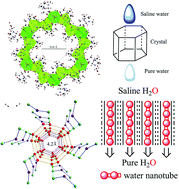
A new type of thermally stable chelate {La(H2O)4[La(1,3-pdta)(H2O)]3}n·12nH2O (1) [1,3-H4pdta![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2[CH2N(CH2CO2H)2]2] with an open-channel shows significant and unusual solvent transport properties and demonstrates a use for low-pressure desalination, which is constructed by cheap and available lanthanum salt and 1,3-propanediaminetetraacetate. The chelate could be converted reversibly to its trihydrate {La(H2O)4[La(1,3-pdta)(H2O)]3}n·3nH2O (1a), dehydrated product {La(H2O)4[La(1,3-pdta)(H2O)]3}n (1b) and ethanol adduct {La(H2O)4[La(1,3-pdta)(H2O)]3}n·3nH2O·3nEtOH (1c). The latter nano-confined ethanol shows a remarkable downfield shift (Δδ = 6.0 ppm) for the methylene group in the solid 13C NMR spectrum compared with that of the free EtOH. Crystal 1 with a regular hexagonal appearance can be used directly for saline water desalination on a small-scale at an ambient temperature, demonstrating a low energy consumption and environmentally friendly method. This is attributed to the 10.0 Å hydrophobic open-channel containing water nanotubes (WNTs, Φ = 4.2 Å). The nano-confined WNTs can be removed at a low temperature (45 °C).
CH2[CH2N(CH2CO2H)2]2] with an open-channel shows significant and unusual solvent transport properties and demonstrates a use for low-pressure desalination, which is constructed by cheap and available lanthanum salt and 1,3-propanediaminetetraacetate. The chelate could be converted reversibly to its trihydrate {La(H2O)4[La(1,3-pdta)(H2O)]3}n·3nH2O (1a), dehydrated product {La(H2O)4[La(1,3-pdta)(H2O)]3}n (1b) and ethanol adduct {La(H2O)4[La(1,3-pdta)(H2O)]3}n·3nH2O·3nEtOH (1c). The latter nano-confined ethanol shows a remarkable downfield shift (Δδ = 6.0 ppm) for the methylene group in the solid 13C NMR spectrum compared with that of the free EtOH. Crystal 1 with a regular hexagonal appearance can be used directly for saline water desalination on a small-scale at an ambient temperature, demonstrating a low energy consumption and environmentally friendly method. This is attributed to the 10.0 Å hydrophobic open-channel containing water nanotubes (WNTs, Φ = 4.2 Å). The nano-confined WNTs can be removed at a low temperature (45 °C).

 Please wait while we load your content...
Please wait while we load your content...