Reduction of hydroxy-functionalised carbaboranyl carboxylic acids to tertiary alcohols by organolithium reagents†
Abstract
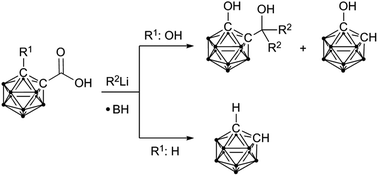
Reduction of hydroxy-functionalised carbaboranyl carboxylic acids by organolithium reagents yields the corresponding tertiary alcohols. This is in contrast to exo-polyhedral C–C bond cleavage of unsubstituted carbaboranyl carboxylic acids upon reaction with lithium organyls. The proposed dimeric contact ion pairs may also explain the formation of tertiary alcohols instead of the expected ketones.
- This article is part of the themed collection: Carboranes

 Please wait while we load your content...
Please wait while we load your content...