Triple-bridged ferromagnetic nickel(ii) complexes: A combined experimental and theoretical DFT study on stabilization and magnetic coupling†
Abstract
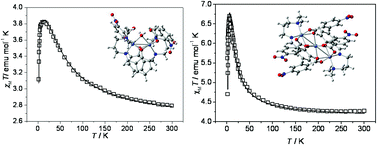
Two dinuclear [Ni2L2(o-(NO2)C6H4COO)2(H2O)] (1), [Ni2L2(p-(NO2)C6H4COO)2(H2O)]·0.5CH3OH (2) complexes and one trinuclear [Ni3L2(p-(NO2)C6H4COO)4]·C2H5OH (3) Ni(II) complex have been synthesized using a tridentate Schiff base ligand, 1-[(3-dimethylamino-propylimino)-methyl]-naphthalen-2-ol (HL) along with ortho- and para-nitro benzoate as co-ligands. All these three (1–3) complexes have been characterized by spectral analysis, X-ray crystallography and variable temperature magnetic susceptibility measurements. The structural analyses reveal that complexes 1 and 2 are dinuclear in which two μ2-phenoxido and a water molecule bridge the two Ni(II) centers to make the complexes face sharing bioctahedra. Complex 3 is a linear triple bridged (phenoxido and carboxylato) trinuclear Ni(II) complex. Variable-temperature magnetic susceptibility studies indicate the presence of ferromagnetic exchange coupling in complexes 1–3 with J values of 25.4, 28.1 and 6.2 cm−1 respectively. Theoretically obtained J values of 21.4 cm−1 (for 1), 22.0 cm−1 (for 2) and 9.3 cm−1 (for 3) corroborate very well the experimental results. DFT calculation also shows that stronger H-bonding interactions present in the case of carboxylate based coligands stabilise the triple oxido-bridged complexes.

 Please wait while we load your content...
Please wait while we load your content...