Crystal structures and DFT calculations of new chlorido-dimethylsulfoxide-MIII (M = Ir, Ru, Rh) complexes with the N-pyrazolyl pyrimidine donor ligand: kinetic vs. thermodynamic isomers†
Abstract
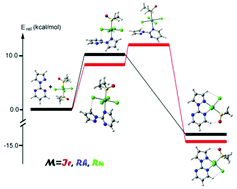
New chlorido-dimethylsulfoxide-iridium(III), ruthenium(III) and rhodium(III) complexes with the 2-(1H-pyrazol-1-yl)-pyrimidine (pyrapyr) ligand (OC-6-N1)-[RhIIICl3(DMSO-κS)(pyrapyr)] (1a, N = 3 and 1b, N = 4); (OC-6-N1)-[RuIIICl3(DMSO-κS)(pyrapyr)] (2a, N = 3 and 2b, N = 4) and (OC-6-N1)-[IrIIICl3(DMSO-κS)(pyrapyr)] (3a, N = 3 and 3b, N = 4) have been synthesized and characterized by spectroscopic techniques and by single crystal X-ray diffraction studies (1a, 1b, 2a, 2b, a disordered crystal 3a/3b and a cocrystal 3a·3b). In all cases, the metal centers show octahedral geometry coordinated to three chloride ligands and one S coordinated dimethylsulfoxide (DMSO-κS). The coordination sphere of the metal is completed by the pyrapyr molecule. Two different coordination modes are observed: (i) the DMSO-κS is opposite to the pyrimidinic N atom (IUPAC nomenclature is OC-6-31 denoted herein as trans); (ii) DMSO-κS is opposite to the pyrazolic N atom (IUPAC nomenclature is OC-6-41 denoted as cis). For Rh(III) the kinetic product (cis) yields the thermodynamic (trans) upon heating a solution of the kinetic product and both isomers have been X-ray characterized. Conversely for Ru(III), both kinetic and thermodynamic complexes have been obtained by using different procedures. Both isomers have been characterized by X-ray crystallography and the kinetic product does not yield the thermodynamic upon heating a solution of the former. Furthermore, the Ir(III) behaves differently, since both isomers are energetically equivalent and both isomers co-crystallize in the solid state. The kinetic/thermodynamic mechanism that yields the different isomers has been studied by using theoretical DFT calculations for each metal. Finally, two Ru(II) complexes (OC-6-N1)-[RuIICl2(DMSO-κS)2(pyrapyr)] (4a, N = 3 and 4b, N = 4) are also described and X-ray characterized. They were obtained as minor products during the synthesis of 2a.

 Please wait while we load your content...
Please wait while we load your content...