Probing the metallating ability of a polybasic sodium alkylmagnesiate supported by a bulky bis(amido) ligand: deprotomagnesiation reactions of nitrogen-based aromatic substrates†
Abstract
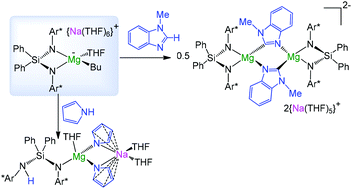
Exploring the reactivity of sodium butylmagnesiate reagent [{Na(THF)6}+{(Ph2Si(NAr*)2)Mg(Bu)(THF)}−] (1) supported by the bulky chelating silyl(bisamido) ligand {Ph2Si(NAr*)2}2− (Ar* = 2,6-iPr2-C6H3) towards N-methylbenzimidazole (bImMe), pyrrole and 2,6-diisopropylaniline (NH2Ar*), this study provides new insights into the ability of this bimetallic base to facilitate direct Mg–H exchange reactions as well as to exhibit polybasicity. Thus 1 effectively promotes the deprotomagnesiation of bImMe under mild reaction conditions to give the α-metallated intermediate [{Na(THF)5}2+{(Ph2Si(NAr*)2)Mg(bImMe*)}2−] (2) (bImMe* = 2-N-methylbenzimidazolyl). Analysis of crystallographic and NMR data of 2 combined with DFT calculations show that the metallated C in the bImMe* ligands possesses a significant carbenic character. Contrasting with previous studies of benzothiazole (btz), 1 does not react with an excess of bImMe even under forcing refluxing conditions. Contrastingly, the amination reactions of equimolar amounts of 1 with pyrrole and 2,6-diisopropylaniline allowed the isolation of [{(Ph2Si(NAr*)(NHAr*))Mg(NC4H4)2(THF)Na(THF)2}] (3) and [{Na(THF)6}+{(Ph2Si(NAr*)(NHAr*))Mg(NHAr*)2(THF)}−] (4) respectively as crystalline solids. Highlighting the ability of 1 to act as a polybasic reagent, 3 and 4 are formed as the result of the deprotonation of two molecules of the relevant amine via its butyl group and one amido arm of the silyl(bisamido) ligand. Similarly, the reactions of 1 with 3 molar equivalents of the relevant amine yielded homoleptic tris(amido) compounds [(THF)2NaMg(NC4H4)3] (5) and [{Na(THF)6}+{Mg(NHAr*)3}−] (7), with the concomitant formation of bis(amine) Ph2Si(NHAr)2, as a result of the complete amination of 1 using its three basic sites. The structures in the solid state of 3 and 4 were elucidated by X-ray crystallography. Despite their similar constitution, these heteroleptic tris(amido)magnesiates adopt contrasting structures, with the former displaying a contacted ion-pair structure, where Na and Mg are connected by two bridging pyrrolyl anions, whereas the latter gives rise to a solvent-separated ion pair motif. To the best of our knowledge 3 represents the first crystallographically characterized magnesium compound containing an anionic non-substituted form of pyrrole. Noticeably, Mg interacts exclusively with the N atoms of the pyrrolyl ligands, forming strong σ-bonds, whereas Na prefers to engage with the π-systems of both NC4-rings.
- This article is part of the themed collection: New Talent: Europe

 Please wait while we load your content...
Please wait while we load your content...