Anion effects on the structures and magnetic properties of binuclear lanthanide single-molecule magnets†
Abstract
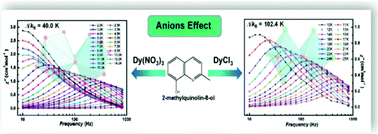
Here we report the anion-induced changes of structures and magnetic properties in binuclear lanthanide compounds. Firstly, two Dy3+-based compounds, [Dy2(Mq)4(NO3)6] (1) and [Dy2(Mq)4Cl6](EtOH)2 (2) (Mq = 8-hydroxy-2-methylquinoline), were synthesized and characterized. They contain similar binuclear Dy2O2 cores, while the different peripheral anions lead to quite different coordination environments of the Dy3+ ion. In compound 1, the Dy3+ ion is nine-coordinated and characterized by a distorted 4,4,4-tricapped trigonal prism environment. In compound 2, the Dy3+ ion has a highly distorted six-coordinated octahedral environment. Their Gd3+ analogues, [Gd2(Mq)4(NO3)6] (3) and [Gd2(Mq)4Cl6](EtOH)2 (4), were also studied to investigate the magnetic interaction between metal ions. Variable-temperature dc magnetic susceptibility measurements show that all the compounds are weakly antiferromagnetically coupled. Ac magnetic susceptibility measurements reveal that both compounds 1 and 2 exhibit single-molecule magnet (SMM) behaviour, while the thermal energy barrier of 2 is significantly higher than that of 1 (Δ/kB = 40.0 K for 1 and Δ/kB = 102.4 K for 2).

 Please wait while we load your content...
Please wait while we load your content...