Accurate tuning of ordered nanotubular platinum electrodes by galvanic plating†
Abstract
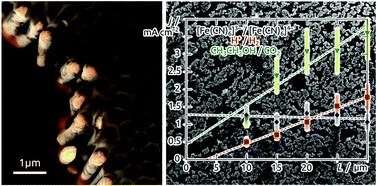
Platinum nanotubes are created by galvanic deposition inside porous templates. The effects of the electrolyte's ion concentration and pH, of the applied potential and of the deposition duration on the morphology of the tubes are investigated systematically. The system provides a model electrode platform with accurately tunable geometry for the fundamental investigation of electrochemical transformations. For slow electrochemical reactions, we observe a linear increase of the galvanic current with the length of the nanotubes, and therefore with the specific surface area of the electrode. In contrast to this, inherently fast electrochemical transformations are diffusion-limited and give rise to the same current density independently of the geometry. These results delineate a strategy for optimizing the performance of electrochemical energy conversion devices systematically via nanostructuring the electrode surfaces.
- This article is part of the themed collection: New Talent: Europe

 Please wait while we load your content...
Please wait while we load your content...