Vibrational spectroscopic studies and DFT calculations on NaCH3CO2(aq) and CH3COOH(aq)†
Abstract
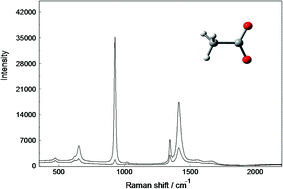
Aqueous solutions of sodium acetate, NaCH3CO2, and acetic acid, CH3COOH, were studied using Raman and infrared spectroscopy. The spectra were recorded over a large concentration range, in the terahertz region and up to 4000 cm−1. In the isotropic Raman spectrum in R-format, a polarized band at 189 cm−1 was assigned to the ν1Na–O stretch of the hydrated Na+-ion and a shoulder at 245 cm−1 to the restricted translation band, νsO–H⋯O* of the hydrated acetate ion, CH3CO2−(aq). The CH3CO2−(aq) and the hydrated acetic acid, CH3COOH(aq), possess pseudo Cs symmetry. Geometrical parameters for the species in the gas phase and for CH3CO2−(aq) and CH3COOH(aq) are reported. Characteristic bands for CH3CO2−(aq) and CH3COOH(aq) were assigned under the guidance of the DFT vibrational frequency calculations and discussed in detail. In aqueous NaCH3CO2 solutions, at high concentrations, no contact ion pairs could be detected, but instead solvent separated ion pairs were found. In LiCH3CO2(aq), however, contact ion pairs are formed which is indicated by the appearance of a shoulder at 939 cm−1 and the shift of the symmetric stretching mode of the –CO2− group to higher wavenumbers.

 Please wait while we load your content...
Please wait while we load your content...