Structural properties of the inner coordination sphere of indium chloride complexes in organic and aqueous solutions†
Abstract
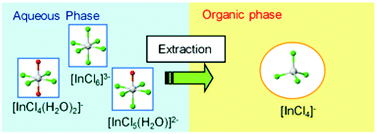
The nature of the inner coordination sphere of In3+ present in both the organic and aqueous solutions during the solvent extraction of In3+ from an aqueous HCl solution with tri-n-octyl amine (TOA) was investigated by In K-edge XAFS. This information was then used to clarify the details of the extraction properties of indium chloride anion complexes with TOA. In aqueous HCl solution (0.1–10 M), In3+ exists as octahedral anion complexes, [InCln(H2O)6−n]3−n (n ≥ 4); the [InCl6]3− complex is dominant at 10 M HCl. The extraction of In3+ from HCl solution with TOA was performed using two kinds of diluents: nitrobenzene (NB) or n-dodecane (DD), which contained 20 vol% of 2-ethylhexanol as an additive. The stoichiometric composition of the extracted complexes, which is estimated from the distribution ratios of In3+, is affected by the diluents and the HCl concentration of the aqueous phase; the apparent values of TOA/In3+ in the extracted complex are 3 for DD–1 M HCl (diluent–aqueous phase) and DD–5 M HCl, 2 for NB–1 M HCl and NB–5 M HCl, and 1 for NB–10 M HCl. The EXAFS analysis of these extracted complexes shows that the In3+ has ∼4 Cl− at ∼2.336 Å and no H2O in the inner coordination sphere; additionally, the shape of the XANES suggests that their coordination geometry is tetrahedral. Therefore, the same tetrahedral [InCl4]− complex is formed during the extraction in spite of the variation in the stoichiometric composition (TOA/In3+ = 1–3) of the extracted complexes.

 Please wait while we load your content...
Please wait while we load your content...