Steric effect involved in Ln3+/Ce3+ exchange in a coordination polymer based on di(2-ethylhexyl)phosphoric acid†
Abstract
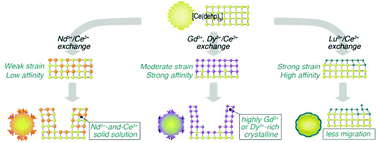
Coordination polymers can be attractive ion exchange materials because of their crystallinity and semi-flexibility, which are rather opposing properties, and play integral and synergistic roles in introducing unique ion-exchange behavior. In this paper, Ln3+/Ce3+ exchange (Ln3+ = Nd3+, Gd3+, Dy3+, or Lu3+) in a coordination polymer, [Ce(dehp)3], based on di(2-ethylhexyl)phosphoric acid (Hdehp) is studied by distribution coefficient measurements, ion-exchange isotherms, Kielland plot analysis, and morphology observation. The ion-exchange selectivity is in the order Nd3+ < Gd3+ < Dy3+ < Lu3+ when a small amount of Ln3+ is loaded, but Lu3+ ≈ Nd3+ < Gd3+ ≈ Dy3+ for a high loading ratio. The Kielland plot suggests that a steric effect is involved in the reactions, which becomes stronger in the order of Nd3+/Ce3+ < Gd3+/Ce3+ < Dy3+/Ce3+ < Lu3+/Ce3+ for exchange systems. This trend is attributable to the differences in the ionic sizes between an incoming Ln3+ and original Ce3+. Scanning electron microscopy observations reveal the generation of a new phase via the Ln3+/Ce3+ exchange. Such a phenomena results from solid–solid transformation, rather than dissolution–recrystallization. The small steric strain in the Nd3+/Ce3+ system leads to the formation of a Nd3+-and-Ce3+ solid-solution, whereas the morphological change is possibly restrained by the strong strain caused by loaded Ln3+ with an ionic size significantly smaller than the original Ce3+.

 Please wait while we load your content...
Please wait while we load your content...