Mechanism of stabilization of dicalcium silicate solid solution with aluminium
Abstract
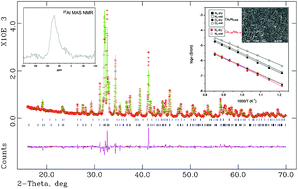
Stoichiometric dicalcium silicate, Ca2SiO4, displays a well-known polymorphism with temperature. When this phase is doped by a range of elements, belite, one of the main phases of cements, is generated. Here, we thoroughly study the aluminum doping of dicalcium silicate. This type of study is important for cement characterization and also from a basic point of view. Ca2Si1−2xAl2xO4−x□x (x = 0, 0.010, 0.014, 0.03) has been prepared and studied by X-ray powder diffraction and the Rietveld method. The limiting composition has been established as Ca2Si0.972Al0.028O3.986□0.014. The 27Al MAS NMR band located close to ∼−70 ppm is ascribed to tetrahedral environments, in agreement with the proposed aliovalent Si/Al atomic substitution mechanism. Thermal analysis measurements under a wet atmosphere indirectly confirm the increase of oxygen vacancies as the amount of incorporated protons increases with the aluminium content. A thorough electrical characterization has been carried out including overall conductivity measurements under wet and dry atmospheres and conductivity as a function of the oxygen partial pressure. The samples show oxide anion conductivity with a small p-type electronic contribution under oxidizing conditions. These compounds display a very important proton contribution to the overall conductivities under humidified atmospheres.

 Please wait while we load your content...
Please wait while we load your content...