Hydrogen-bond-supported dimeric boron complexes of potentially tetradentate β-diketiminate ligands†
Abstract
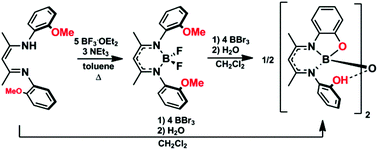
Two dimeric boron complexes of potentially tetradentate and trianionic β-diketiminate ligands bearing phenol substituents were prepared and characterized. The synthetic routes employed were designed to circumvent the undesirable formation of β-ketimines and 2-methylbenzoxazoles observed when traditional synthetic routes toward the target β-diketiminate ligands were attempted. The title complexes were isolated via demethylation of β-diketimine ligands and boron difluoride complexes bearing 2-anisole N-aryl substituents using boron tribromide. The resulting complexes were found to contain a unique hydrogen-bond-supported boron–oxygen–boron bridge, as confirmed by X-ray crystallography. The stability of the resulting dimeric structures relative to the corresponding monomeric, tetradentate boron complexes was studied computationally, and theory confirmed that the dimeric structures were strongly favored. The absorption spectra of the dimers were red-shifted relative to the parent β-diketimine ligands. The complexes were irreversibly oxidized and reduced electrochemically and were weakly emissive at low concentrations (Stokes shifts between 23 and 31 nm), showing little solvent dependence.

 Please wait while we load your content...
Please wait while we load your content...