A spectroscopic study on the coordination and solution structures of the interaction systems between biperoxidovanadate complexes and the pyrazolylpyridine-like ligands†
Abstract
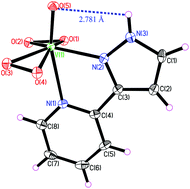
In order to understand the substitution effects of pyrazolylpyridine (pzpy) on the coordination reaction equilibria, the interactions between a series of pzpy-like ligands and biperoxidovanadate ([OV(O2)2(D2O)]−/[OV(O2)2(HOD)]−, abbrv. bpV) have been explored using a combination of multinuclear (1H, 13C, and 51V) magnetic resonance, heteronuclear single quantum coherence (HSQC), and variable temperature NMR in a 0.15 mol L−1 NaCl D2O solution that mimics the physiological conditions. Both the direct NMR data and the equilibrium constants are reported for the first time. A series of new hepta-coordinated peroxidovanadate species [OV(O2)2L]− (L = pzpy-like chelating ligands) are formed due to several competitive coordination interactions. According to the equilibrium constants for products between bpV and the pzpy-like ligands, the relative affinity of the ligands is found to be pzpy > 2-Ester-pzpy ≈ 2-Me-pzpy ≈ 2-Amide-pzpy > 2-Et-pzpy. In the interaction system between bpV and pzpy, a pair of isomers (Isomers A and B) are observed in aqueous solution, which are attributed to different types of coordination modes between the metal center and the ligands, while the crystal structure of NH4[OV(O2)2(pzpy)]·6H2O (CCDC 898554) has the same coordination structure as Isomer A (the main product for pzpy). For the N-substituted ligands, however, Isomer A or B type complexes can also be observed in solution but the molar ratios of the isomer are reversed (i.e., Isomer B type is the main product). These results demonstrate that when the N atom in the pyrazole ring has a substitution group, hydrogen bonding (from the H atom in the pyrazole ring), the steric effect (from alkyl) and the solvation effect (from the ester or amide group) can jointly affect the coordination reaction equilibrium.

 Please wait while we load your content...
Please wait while we load your content...