Radiation induced physicochemical changes in FAP (fluoro alkyl phosphate) based imidazolium ionic liquids and their mechanistic pathways: influence of hydroxyl group functionalization of the cation†
Abstract
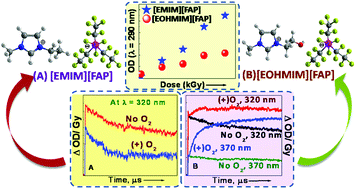
Future applications of ionic liquids (ILs) in a variety of areas, especially those having high radiation fields such as the nuclear fuel cycle and in space technology, are under serious consideration nowadays. For such applications to be possible, however, radiation stability of the ILs is an important issue that needs to be addressed. We envisaged that the ultra-hydrophobic, bulky and hydrolytically stable FAP (tris(perfluoroalkyl)trifluorophosphate) anion might shield the radiolytically vulnerable imidazolium cations from degradation and our result shows that these anions indeed enhance their radiolytic stability. However, introduction of a hydroxyl group into the alkyl side chain of the imidazolium moiety resulted in significant changes in the physical properties of the IL with respect to onset temperatures, conductivity and the electrochemical window. Furthermore, a nonlinear trend in absorbance with an increase in radiation dose accompanied by NMR (nuclear magnetic resonance) and mass spectrometry studies clearly demonstrated that the presence of the hydroxyl group promotes various degradation channels. Interestingly, a perturbation of the hydrogen bond between the hydroxyl group (present in the side chain of the cation) and the fluorine atom of the anion (OH⋯F) was evident in the case of irradiated hydroxyl functionalized FAP ILs. Besides, the hydrogen gas yields of the ILs were determined and found to be comparable to those of a radiolytically stable aromatic compound, benzene. Finally, through transient spectroscopic studies we could delineate the mechanism of the radiation induced changes in the physicochemical properties of the non-hydroxyl and hydroxyl containing FAP ILs. We have clearly demonstrated that a simple functionalization of the molecular structure of the FAP based imidazolium ILs might cause marked differences in the reactivity, reaction center and the nature of the radiolytic products, which eventually lead to significant changes in their physicochemical properties.

 Please wait while we load your content...
Please wait while we load your content...