Dehydration induced 2D-to-3D crystal-to-crystal network re-assembly and ferromagnetism tuning within two chiral copper(ii)–tartrate coordination polymers†
Abstract
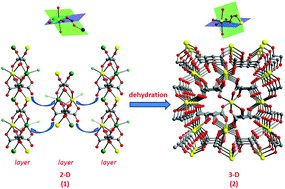
The synthesis of two homochiral L-tartrate–copper(II) coordination polymers, [Cu2(C4H4O6)2(H2O)2·xH2O]n (1), and [Cu(C4H4O6)]n (2), under hydrothermal conditions, is reported. Compound 1 adopts a 2D layered network structure with a space group of P21, while compound 2 features a 3D network structure with a space group P21212. Interestingly, the 2D layered structure of compound 1 can undergo a crystal-to-crystal network reassembly, with the formation of the 3D network structure of compound 2 under dehydration conditions. Variable temperature and field magnetic studies reveal the existence of a distinct ferromagnetic interaction between Cu2+ ions as the result of distinct syn–anti carboxylate bridging coordination modes.

 Please wait while we load your content...
Please wait while we load your content...