Di(imino)aryltin(iv) dichlorides as tectons for heterometallic coordination compounds†
Abstract
Hydrolysis of [2-{(CH2O)2CH}C6H4]2SnCl2 (1) [prepared from 2-[(CH2O)2CH]C6H4MgBr and SnCl4, in 2 : 1 molar ratio] gave [2-(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C6H4]2SnCl2 (2). Treatment of 2 with the appropriate amine, in the absence of a solvent or catalyst, resulted in the isolation in high yields of [2-(RN
CH)C6H4]2SnCl2 (2). Treatment of 2 with the appropriate amine, in the absence of a solvent or catalyst, resulted in the isolation in high yields of [2-(RN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C6H4]2SnCl2 [R = 2′-C10H7 (3), 2′,4′,6′-Me3C6H2 (4), PhCH2 (5), Me2NCH2CH2 (6), 2′-PyCH2 (7)]. The reaction of 7 with [Pd(COD)Cl2] provided the heterometallic species [Cl2Pd{2-(2′-PyCH2N
CH)C6H4]2SnCl2 [R = 2′-C10H7 (3), 2′,4′,6′-Me3C6H2 (4), PhCH2 (5), Me2NCH2CH2 (6), 2′-PyCH2 (7)]. The reaction of 7 with [Pd(COD)Cl2] provided the heterometallic species [Cl2Pd{2-(2′-PyCH2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C6H4}2SnCl2] (8). The compounds were characterised by multinuclear NMR spectroscopy in solution, and mass spectrometry and IR spectroscopy in the solid state. The molecular structures of 1, 2, 4–7 and 8·CH3CN were established by single-crystal X-ray diffraction. For all diorganotin(IV) dichlorides intramolecular O→Sn or Nimine→Sn coordination results in hypercoordinated species with a distorted octahedral (C,E)2SnCl2 core (E = O, N). The presence of intramolecular N→Sn interactions in solution, responsible for the restriction of free rotation of mesityl groups around the C–N(
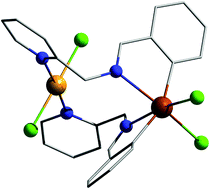
CH)C6H4}2SnCl2] (8). The compounds were characterised by multinuclear NMR spectroscopy in solution, and mass spectrometry and IR spectroscopy in the solid state. The molecular structures of 1, 2, 4–7 and 8·CH3CN were established by single-crystal X-ray diffraction. For all diorganotin(IV) dichlorides intramolecular O→Sn or Nimine→Sn coordination results in hypercoordinated species with a distorted octahedral (C,E)2SnCl2 core (E = O, N). The presence of intramolecular N→Sn interactions in solution, responsible for the restriction of free rotation of mesityl groups around the C–N(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) single bonds, is suggested by 1H and 13C NMR data. In the heterometallic dinuclear complex 8 the octahedral coordination around tin is preserved as in 7 and the nitrogen atoms from the pyridyl groups in the pendant arms are coordinated to palladium, leading to a trans-square planar PdCl2N2 core.
C) single bonds, is suggested by 1H and 13C NMR data. In the heterometallic dinuclear complex 8 the octahedral coordination around tin is preserved as in 7 and the nitrogen atoms from the pyridyl groups in the pendant arms are coordinated to palladium, leading to a trans-square planar PdCl2N2 core.

 Please wait while we load your content...
Please wait while we load your content...