A regeneratable and highly selective fluorescent probe for sulfide detection in aqueous solution†
Abstract
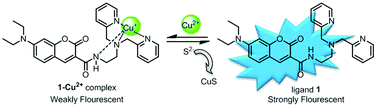
A novel fluorescent probe based on a coumarin–dipicolylamine–Cu2+ complex was synthesized and characterized. The probe is able to highly sensitively and selectively sense sulfide over other common anionic analytes including thiols followed by releasing ligand 1 to give a remarkable change of fluorescent intensity in aqueous solution. The probe could be regenerated by the addition of Cu2+, and therefore the probe could be utilized repetedly to sense sulfide anions with Cu2+ and S2−, in turn, increased. The detection limit of the fluorescent assay for sulfide is as low as 14 nM with a rapid response time (a few seconds). Importantly, the displacement mechanism for sensing of sulfide anions was clarified through single-crystal X-ray diffraction analysis and DFT calculations. Based on this probe, a rapid, selective and sensitive fluorescence method for the detection of sulfide anions in environmental samples was established.

 Please wait while we load your content...
Please wait while we load your content...