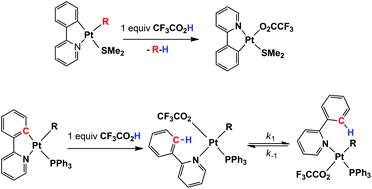
Reaction of each of the known starting complexes [PtR(C^N)(SMe2)], 1, in which R = Me or p-MeC6H4 and C^N is either ppy (deprotonated 2-phenylpyridine) or bhq (deprotonated benzo[h]quinoline), with one equivalent of CF3CO2H, gave the complexes [Pt(C^N)(CF3CO2)(SMe2)], 3 (C^N = ppy, 3a; bhq, 3b). The bis-chelate complexes [Pt(C^N)(P^P)](CF3CO2), 4, were obtained by reaction of complexes 3 with one equivalent of either of the P^P bisphosphine reagents, dppf = 1,1′-bis(diphenylphosphino)ferrocene or dppe = bis(diphenylphosphino)ethane. Complexes 4 were alternatively made by reaction of the complexes [PtMe(κ1C-C^N)(P^P)], 2, with one equivalent of CF3CO2H. When the complex 3b was reacted with 0.5 equivalents of dppe, 0.5 equivalents of the related bis-chelate product, 4d, formed along with 0.5 equivalents of the unreacted starting complex 3b. In contrast, when the complex 3b was reacted with 0.5 equivalents of dppf, then the dimeric complex [Pt2(bhq)2(CF3CO2)2(μ-dppf)], 5, formed in pure form. In all the above-mentioned acid reactions, the M–R bond rather than the M–C bond of the cycloplatinated complex is cleaved. When the PPh3 analogues of complexes 1, i.e. the complexes [PtR(C^N)(PPh3)], 6, in which C^N is ppy or tpy = deprotonated 2-p-tolylpyridine, were reacted with one equivalent of CF3CO2H, the course of the reaction reversed and the M–C bonds of the cycloplatinated complexes are cleaved rather than the M–R bonds. The latter reaction gave [PtR(κ1N-HC^N)(PPh3)(CF3CO2)], as an equilibrium mixture of two isomers 7 and 8. Crystal structures of the typical complexes show a variety of extensive intermolecular hydrogen bonding involving C–H bonds from the different ligands and electronegative atoms (O or F) from the CF3CO2 moiety. On the basis of data obtained from kinetic studies (using 1H NMR spectroscopy), a dissociative mechanism is proposed for the case of the 7c/8c isomerization process, involving dissociation of the κ1N-Htpy neutral ligand, rather than the alternative route of PPh3 or CF3CO2 ligand dissociation.

 Please wait while we load your content...
Please wait while we load your content...